Characterization of RNAdvance Viral XP RNA Extraction Kit using AccuPlex™ SARS–CoV–2 Reference Material Kit
Content Type: White PaperAuthors: Wenjing Pan1, Brittany Brown1, Jung Doh2, Miranda Byrne–Steele1
Companies: 1iRepertoire Inc., Huntsville, AL, 2Beckman Coulter Life Sciences, Indianapolis, IN
Abstract
Recently, a world–wide shortage of viral RNA extraction kits has developed due to a recent health crisis that has diverted supply chains away from supporting extraction kits for research use. To help alleviate this issue, Beckman Coulter has introduced a new RNA extraction kit, the RNAdvance Viral XP kit for research use. This new RNA extraction kit utilizes Beckman Coulter, Inc.’s Solid Phase Reversible Immobilization (SPRI) paramagnetic bead–based technology to isolate viral RNA from nasopharyngeal/ oropharyngeal swabs. iRepertoire and Beckman Coulter worked closely and rapidly to characterize the functional capabilities of the kit. iRepertoire performed analytical performance assays and specimen assays to demonstrate that the RNAdvance Viral XP extraction kit has a limit of detection of RNA (LoD) of 1 copy of RNA, specifically tested with AccuPlex™ SARS–CoV–2 Reference Material Kit, per microliter of sample. Through a series of specimens, we further demonstrate that the extraction results are not adversely affected by the Universal Transport Media used to collect SARS–CoV–2 specimens. Even for research use only purposes, it is important to demonstrate performance characteristics demonstrated in this paper. The results clearly demonstrate that Beckman Coulter’s RNAdvance Viral XP RNA extraction kit represents a viable alternative to help alleviate a critical shortage in viral RNA extraction kits for research use.Introduction
RNA extraction is the first step in performing qRT–PCR–based methods for the determining the presence of virus in samples. However, many extraction kits and materials are in short supply. To help alleviate the global supply chain for RNA extraction kits, Beckman Coulter introduced a novel viral extraction kit for research use, the RNAdvance Viral XP kit.
The RNAdvance Viral XP kit utilizes Beckman Coulter, Inc.’s Solid Phase Reversible Immobilization (SPRI) paramagnetic bead–based technology to isolate viral RNA from nasopharyngeal/oropharyngeal swabs. The protocol can be performed in both 96–well plate and single tube formats. Purification begins with lysis of the viral capsid. Following lysis, the RNA is immobilized onto the magnetic particles allowing separation from contaminants using a magnetic field. The contaminants are then rinsed away using a simple wash procedure and an elution buffer is used to release the RNA from the magnetic beads. The protocol defined for this product enables isolation of viral RNA from 200μL of viral transport media per well in 96–well plate and 1.5 mL tube formats. Using manual extraction, up to 48 samples per run can be processed to extract RNA in 45 mins with hands on time of 25 mins. RNA extracted by this method is compatible for use with research methods using RT PCR.
Here, we report a series of experiments that demonstrate the analytical performance of the assay and its performance in the context of 30 nasopharyngeal specimens collected from healthy donors. Since no quantified virual isolates of the SARS-CoV–2 are currently available, SeraCare RNA Reference Material (AccuPlex™ SARS–CoV–2 Reference Material Kit ) of known titer (RNA copies/μL) were used for positive and negative specimens. SeraCare standard contains viral RNA enclosed in a capsid making it an accurate standard for viral workflows. First, we performed an experiment to ascertain the likely limit of detection of RNA (LoD) of the assay, which was defined as the concentration of SeraCare positive RNA at which 95% or greater detection was observed. Next, we performed a analytical sensitivity assay with 20 replicates each at the putative LoD (1 copy/μL), 3–fold higher (3 copies/μL), and 3–fold lower (0.3 copy/μL). Finally, we performed an evaluation whereby we utilized 30 nasopharyngeal flock swabs in Universal Transport Media (UTM). SARS–CoV–2 negative and positive specimens were from the donor UTM, extracted, and tested with the IDT’s 2019–nCoV CDC RUO Primers and Probes (PN: 10006713).
Materials and Methods
Analytical Performance
Input concentrations of 0.8, 1, 1.5, and 2 copies/μL of the SeraCare RNA Reference Material (AccuPlex™ SARS–CoV–2 Reference Material Kit) were spiked into PBS containing ~5,000 lung cancer epithelial cells. RNA was extracted using the RNAdvance Viral XP Kit in triplicates. For negative extraction controls, 1 copy/μL of the SeraCare Negative Controls were spiked into PBS containing an additional ~5,000 lung cancer epithelial cells, and extractions were performed in duplicates. The additional cells were added to more closely mimic real sample background. Ct values were assessed via qRT–PCR (Biorad’s Reliance One–Step Multiplex kit) using a Mic qPCR instrument (Bio Molecular Systems), and 2019–nCoV CDC RUO Primers and Probes (PN: 10006713) for the detection of viral RNA and the RNase P (NP) primer set for the detection of human RNase P RNA (Integrated DNA Technologies).
The analytical sensitivity was verified by extracting twenty individual SeraCare positive control samples at a concentration of 0.3 copy/μL, twenty at 1 copy/μL (estimated limit of detection of RNA (LoD)), and twenty at 3 copy/μL of positive control SeraCare reference. In addition, twenty SeraCare Negative Reference Material for targeting sequences for the human RNase P gene at 1 copy/μL were also extracted. To generate background which more closely mimics real samples, all test samples were spiked with lung cancer epithelial cells at ~5,000 cells per each 200μL sample. Additionally, two water control samples were included from extraction through detection. Samples were extracted using the RNAdvance Viral XP kit. qRT–PCR was performed on each extracted sample using the 2019–nCoV CDC RUO Primers and Probes (PN: 10006713). For each specimen, 3 reactions were run including N1, N2, and RP primers and probes. Additionally, positive and negative controls were run to ensure proper testing control.
Specimen Performance Across Different Concentrations of RNA
Specimen performance was demonstrated on 30 samples containing a known quantity of SeraCare RNA Reference Material and 30 samples with SeraCare Negative Reference Material. Specimens were prepared by spiking SeraCare RNA Reference Material (AccuPlex™ SARS–CoV–2 Reference Material Kit) into Universal Transport Media (UTM) resuspended with nasopharyngeal swabs from healthy human volunteers. Out of the 30 samples containing a known quantity of SeraCare RNA Reference Material, 10 were at a concentration of 1 copy/μL, 10 were at a concentration of 2 copy/μL, 5 were at a concentration of 5 copy/μL and 5 were at a concentration of 10 copy/μL. The 30 samples with SeraCare Negative Reference Material were prepared similarly by spiking in 1 copy/μL of SeraCare Negative Reference Material into Universal Transport Media (UTM) resuspended with nasopharyngeal swabs from healthy human volunteers.
RNA from all the samples were extracted using RNAdvance Viral XP kit. In addition, two water controls were also included in the RT–PCR assay. RT–PCR assays were performed according to 2019–nCoV CDC RUO Primers and Probes (PN: 10006713) instructions for use. For each specimen, 3 amplifications were run including N1, N2, and RP genes. Additionally, the positive and negative controls for the Real–Time RT–PCR probe assay were run to ensure proper testing control.
Results
Samples were accepted based on the Ct value calculated by the MIC qPCR software. Samples were deemed positive if the fluorescence trace shows an exponential increase in fluorescence consistent with a working qPCR and if the Ct value was 40 or lower. Samples were deemed negative if the Ct value either could not be calculated or was higher than 40.
For the estimation of the limit of detection of RNA (LoD), the results indicate the LoD of the RNAdvance Viral XP kit to be 1 copy/μL with an average Ct value of 36. All negative controls gave a Ct value of not determined (ND). The data is summarized in Table 1. Although the input of 0.8 copies/μL had 100% detection for all 3 tested portions of the N–gene, the average Ct values for N3 were approaching the positive (LoD) of a Ct of 40 with three replicates. Therefore, we set the putative LoD at 1 copy/μL for the verification experiments.
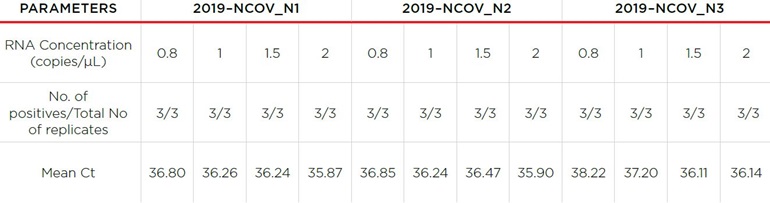
Table 1. Summary of limit of detection of RNA estimation experiments. All negative samples were ND as expected.
For the analytical sensitivity experiments, we tested a higher number of replicates 3–fold both above and below the putative LoD. In order for a specific concentration of positive specimen to qualify for the LoD, we required at least ≥ 95% (19/20) 1xLoD (1 copy/μL) positive samples to have Ct <40, all samples with LoD greater than 1 must have Ct<40, 0/20 negative samples must have Ct <40, and 0/2 water samples must all have Ct <40. As summarized in Table 2, all the contrived positive samples across analytical range were determined to be positive with a Ct less than 40. All the contrived non–reactive samples were determined to be negative with no amplification of PCR. Three–fold below the LoD, we began to observe replicates fall out of range. Since all samples at and above 1 copy/μL were positive, the LoD of the extraction kit is verified to be 1 copy/μL.
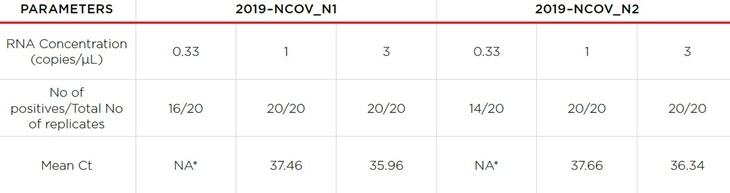
Table 2. Summary of analytical sensitivity experiments. All negative samples were ND as expected.
Specimen performance was demonstrated on 30 samples containing a known quantity of SeraCare RNA Reference Material and 30 samples with SeraCare Negative Reference Material. All the samples containing a known quantity of SeraCare RNA Reference Material across analytical range were determined to be positive per PCR amplification with a Ct value less than 40. All the samples containing SeraCare Negative Reverence Material were determined to be negative per no PCR amplification. Results of the study are summarized in Table 3. Complete data set for the performance study is available upon request.
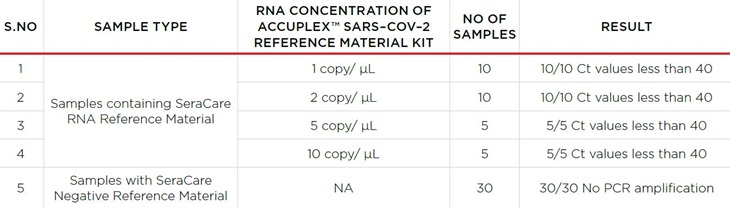
Table 3. Summary of specimen performance across different concentrations of RNA experiments. All negative samples were ND as expected.
Discussion
One of the first steps in processing samples for research of viruses is the RNA extraction step. Unfortunately, there is a worldwide supply issue with regards to available kits, and there is a need for additional validated kits in the market. Here, we show that the RNAdvance Viral XP extraction kit is a good option for the extraction step. The workflow for the RNAdvance Viral XP extraction kit is straightforward as shown in Figure 1. The basic procedure involves a lysis step, immobilization of nucleic acid on magnetic beads, cleaning of contaminants, and elution of the purified nucleic acid from the bead. Here, we demonstrated that this RNA extraction kit can very successfully detect down to 1 copy/μL of SARS–CoV–2 viral RNA in contrived specimens.

Figure 1. Overview of the Beckman Coulter RNAdvance Viral XP extraction kit process.
1. Add Lysis LBF to viral transport media and mix
2. Addition of Bind VBE
3. Magnetic separation of beads from supernatant, wash with Ethanol
4. Elution
An additional benefit to bead–based methods is that they lend themselves readily to automated platforms. The RNA extraction can be a laborious and time–consuming step. Thus, methods that reduce the labor and overall time to result will increase the throughput of testing. Although the experiments performed here were done manually, the option for automation is an alluring advantage. The parallel processing of 96– or 384–samples on liquid handlers could greatly increase the throughput of many laboratories. The results from this effort clearly demonstrate that Beckman Coulter’s RNA Advance Viral XP RNA extraction kit represents a viable alternative to help alleviate a critical shortage in the SARS CoV–2 research supply chain.
Beckman Product Information: Viral RNA Extraction Reagent Kits
|
RNAdvance Viral Reagent Kit - 768 Preps |
RNAdvance Viral XP Reagent Kit - 1056 Preps |
For research use, not intended for diagnostic purposes.
Related Webinar
Watch the collaboration between Beckman Coulter Life Sciences and Integrated DNA Technologies (IDT) for the solutions to qPCR sample prep, featuring viral RNA extraction reagent kits.
Learn More About
RNAdvance Viral
Research Reagents
Helpful Links:
Extraction Kits:
RNAdvance Viral
RNAdvance Viral XP
Performance Data
Genomic Automated
Workstations:
PCR Purification
RNA/DNA Extraction



